
Importance of cannabis terpenes
We would like to focus on this topic for several reasons. In the first place, for the proven benefit those terpenes give us, and secondly, what would be the meaning of a flower without aroma…
Iberian Peninsula express shipping
0€ Orders over 60€
*6€ orders under 59€
East, West, South Europe express shipping
0€ Orders over 150€
*18€ orders under 149€
North Europe express shipping
0€ Orders over 160€
*20€ orders under 159€
Ireland, Norway, UK normal shipping
0€ Orders over 60€
*6€ orders under 59€
Rest of the world normal shipping
0€ Orders over 180€
*25€ orders under 179€
Shipping costs can be confirmed in your shopping cart.
For additional shipping methods, please reach out through info@kannabia.com.


*Website protected by SSL.
**Not available in all regions.
*Your coupon will be sent via email.
*Should you have any question, comment or feedback, please do not hesitate to contact us.
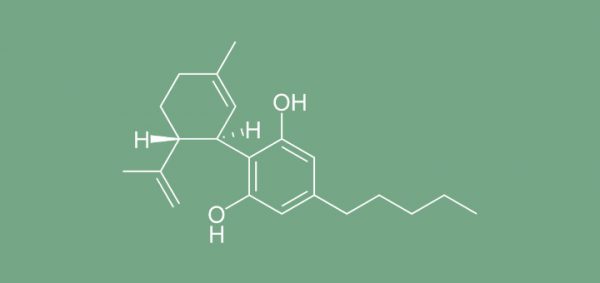
The manufacturer of the CBD extract Epidiolex, GW Pharmaceuticals, reported of positive results of the second placebo-controlled Phase 3 clinical trial of this preparation for the treatment of seizures associated with Lennox-Gastaut syndrome, a rare and severe form of childhood-onset epilepsy. The trial randomized 225 patients into three arms, where Epidiolex was added to the current treatment in a dose of 20mg/kg body weight per day (n=76) or in a dose of 10mg/kg/day (n=73). The other patients (n=76) received a placebo.
Epidiolex Cannabidiol
Patients taking Epidiolex in the higher dose achieved a median reduction in monthly drop seizures of 42 percent compared with a reduction of 17 percent in patients taking placebo, and patients taking Epidiolex in the lower dose achieved a median reduction in monthly drop seizures of 37 percent. “The positive outcome in this second trial of Epidiolex in patients with Lennox-Gastaut syndrome demonstrates the effectiveness of this product in this particularly difficult to treat, childhood-onset epilepsy,” stated Dr Orrin Devinsky of New York University Langone Medical Centers and principal investigator in the trial.
Ensayos clínicos
Comunicado deprensa de GW Pharmaceuticals del 26 de septiembre de 2016.
(*) Photo:Wikimedia Commons